The emergence of multicellular organisms is regarded as one of the major transitions in evolution (Maynard Smith & Szathmáry, 1995). During this evolutionary transition some individual cells `gave up' part of their autonomy to become differentiated functional units of multicellular assemblages. In so doing, the organisms had to develop ways of organising themselves and communicating with each other over long distances. This thesis focuses on the process of multicellular coordination.
The three main types of multicellularity evolved independently from different kinds of unicellular Protista. An explosion of metazoan morphotypes took place some 540 million years ago, during the early Cambrian. This was followed about 40 million years later by the appearance of large numbers of multicellular plants and fungi. Multicellular organisms, however, are even older. Ediacaran faunas date back at least 565 million years, and there are even indications of pre-Ediacaran multicelled creatures (Conway Morris, 2000). A transition from unicellularity to a simple type of multicellularity is still occurring today and can be studied in the cellular slime mould Dictyostelium discoideum, which is the `model' organism investigated in this thesis.
Dictyostelium is a unicellular eukaryote, and therefore belongs to the kingdom of Protista. Nevertheless, once in many generations this unicellular amoeboid suddenly turns into a multicellular creature, a transition which is triggered by food shortage. After the transition, the organism is able to sense new cues from the external world, such as light, temperature- and pH-gradients and air-currents, and the behaviour of the whole organism adjusts to these signals in a coordinated way. Many other species of unicellular organisms can also convert to cooperative behaviour. This usually occurs when they are subjected to harsh conditions, and is often attended by complex pattern formation. Collective behaviour can for example be observed in the bacteria Escherichia coli (Budrene & Berg, 1991,1995), Bacillus subtilis (Kawasaki et al., 1997) and Proteus mirabilis (Matsuyama et al., 2000), and the yeast Saccharomyces cerevisiae (Palková et al., 1997). Nevertheless, the cellular slime moulds are considered to be the most primitive organisms which have all the basic elements of multicellular development (Maeda, 2000).
Because in Dictyostelium the transition from unicellularity to multicellularity can easily be triggered in experimental settings and is not accompanied by cell divisions, a detailed study of this organism gives us the opportunity to find out how multicellularity can develop and how a `fresh' multicellular `organism' can sense the environment and form a collective and appropriate response. This opportunity has been embraced by scientists with research interests in fields such as gene regulation, signal transduction, pattern formation and morphogenesis (Maeda et al., 1997). As a consequence, Dictyostelium has been the subject of many detailed studies, and large amounts of information have been and still are being produced. However, to understand how these processes work, it is important to combine all this information. This can be done using a method of modular modelling. In the second part of this thesis we present a comprehensive model which describes the multicellular coordination and development of Dictyostelium.
A great challenge for those engaged in biological modelling is to find simplifications without taking interesting biological details for granted. Both experimental observations and theoretical evolutionary studies have shown that in general organisms do not evolve minimal solutions. This means that it is important to retain enough biology in the model. In this thesis our strategy is to develop the model in terms of a low level coupling between a few modules. The two main modules in the model are a description of cell signalling in the form of an excitable medium, and a description of cell rearrangement by means of differential adhesion. The two modules have been well established experimentally in isolation and have also been well studied theoretically. Our modelling approach allows us to tightly integrate physical aspects of morphogenesis with cell signalling and cell differentiation; this integration seems to be essential if the model is to reproduce the intricate patterns, forms and dynamics that we observe in nature.
The model can describe how Dictyostelium discoideum morphogenesis unfolds: previously, Savill & Hogeweg (1997) described how single cells develop into a crawling slug; in this thesis we describe how the slug then orientates towards the light and/or responds to very shallow and very noisy temperature gradients, and how it finally transforms itself into a fruiting body, i.e. a globule of spores on a slender stalk. By means of our model we can explain the mechanisms which lead to the normal morphogenesis as well as to aberrant phenotypes. And although in the course of this thesis we increase the complexity of the model, extensions never invalidate previous results; instead we show that self-organisation continues and drives the global dynamics.
In this Introduction we will first explain the title `from pattern formation to morphogenesis'; next we will present a detailed background for the two main modules we use; we continue with an extensive survey of Dictyostelium; and finally we give an overview of the whole thesis.
The formation of intricate patterns `from almost nothing' is a fascinating area of scientific studies. It is known that several generic mechanisms can generate complex patterns via simple rules. The prime examples of these are excitable media (see, e.g. Murray, 1993) and Turing patterns (Turing, 1952). The striking resemblance between patterns produced by such mechanisms and patterns observed in nature, combined with the fact that the underlying dynamics could be very general, has tempted theoreticians to explain biological development in terms of such patterns.
Turing (1952) was the first to study the problem of pattern formation relating to development. He showed how periodic stationary patterns can emerge spontaneously in a homogeneous system, and pointed out that this could be important for the breaking of symmetry during morphogenesis (see also Chaplain et al., 1999). Turing patterns are used to explain many patterns, including the coats of animals, e.g. zebra stripes. Initial variations in the size and shape of the medium, as well as in the saturation-levels of the reaction-part of the model, literally lead to a whole `zoo' of patterns (Murray, 1993).
Note, however, that explanations for early segmentation in the fruitfly Drosophila based on this minimal model (Kauffman, 1981; Kauffman et al., 1978) have been falsified: the formation of each segment is regulated differently (Nusslein-Volhard, 1991; Akam, 1987). Thus, it is important to look at other features besides spots and stripes. In fact, in no biological system have the morphogens involved in this type of pattern formation been identified1.
The other prime pattern formation mechanism is excitable media, which is the main pattern-generating mechanism studied in this thesis. Fortunately, the situation here is entirely different: for many systems the underlying physiology or chemistry of excitability has been well established. This is also the case for Dictyostelium cAMP signalling. In section 1.4 we describe the mechanism of excitability, and we elucidate some examples of models which represent Dictyostelium as an excitable medium.
The `classical' view of development by pattern formation is based on the assumption that a (more or less) static prepattern is established and `read out' by the cells, which respond to it by differentiating, migrating or changing their shape. That is, once the morphogen prepattern is defined, morphogenesis is a slave process (Murray & Swanson, 1999). Not only Turing patterns, but also gradient models for `positional information' (Wolpert, 1969), are classic examples of such chemical prepattern models. In this thesis we do not decouple pattern formation and morphogenesis. Instead, we use the word morphogenesis in a wide sense involving all aspects of development. To understand how the morphogenesis of Dictyostelium is coordinated, we need to know about: (i) the formation and dynamic changes not only of patterns, but also of shapes; (ii) morphogenetic movements on different scales; and (iii) the interplay of the organism with the environment.
We consider morphogenesis as an inherently multilevel process, involving processes on different time and space scales. Here we focus on the entanglement between these levels: we consider not only how microlevel `rules' give rise (via a self-structuring process) to macrolevel behaviour (as in pattern formation models), but also how the macrolevel behaviour determines the microlevel behaviour. This reciprocal influence between the levels can be considered as an essential characteristic of living systems (Hogeweg, 2000b). Thus, in our model morphogenesis is no longer a slave process, but unfolds by the interactions between pattern formation, the collective behaviour of the cells, and its feedback to the pattern formation process. We observe new features on many different scales. As a result, our model leads to explanations of morphogenesis in terms of mechanisms not directly included in the model formulation, but which emerge from the model at an intermediate level (e.g. the pressure waves and peristalsis, which drive the stalk downwards during culmination, see chapters 5 and 6).
In conclusion, we will develop a comprehensive model which is not restricted to explaining pattern formation but incorporates many aspects of Dictyostelium morphogenesis. This means that we move one step beyond `equating' a Turing pattern with a zebra. When asked if his theory of morphogenesis could account for the stripes on a zebra, Turing allegedly responded: ``The stripes are easy, it's the horse part that troubles me!''.
Pattern formations in excitable media are important examples of self-organisation phenomena in spatially distributed biological and chemical systems. Excitable media have been studied for a long time, in for example heart tissue (Allessie et al., 1973), the Belousov-Zhabotinsky (BZ) reaction (Winfree, 1972) and cellular slime moulds (Durston, 1973).
An excitable medium is a medium which has two main properties, namely the ability to conduct pulses of excitation, and the ability to recover its properties after some period of time, called the refractory period. Excitable media can be regarded as a generic concept for pattern formation: it consists of a whole class of systems, which have to possess these few basic properties in order to produce the same common generic behaviour. It is a form of abstraction at the process level, i.e. it defines a generic class of interactions. Obviously, such interactions can be realized in many different ways. For example, Martiel & Goldbeter (1987) have worked out in some detail the receptor kinetics in Dictyostelium, which lead it to behave as an excitable medium (see section 1.4.3).
There are two main classes of excitable media, namely relaying excitable media and oscillatory excitable media. Examples of systems that behave like relaying excitable media include waves of electrical stimulation propagating through cardiac tissue (Panfilov & Holden, 1997) and reverberating cortical depression waves in the brain cortex (Shibata & Bures, 1972); many typical examples of oscillatory excitable media can be found in more ecological contexts, e.g. in the spruce budworm dynamics (Ludwig et al., 1979), hypercycles during pre-biotic evolution (Boerlijst & Hogeweg, 1991), and predator-prey waves (Savill & Hogeweg, 1999). Some examples in which both types of dynamics can be observed are the two- and three-dimensional waves in the BZ-reaction (Winfree & Strogatz, 1984; Jahnke et al., 1988), spiral calcium waves propagating on the surface of an amphibian's egg (Lechleiter et al., 1991; Vaessen, 1999) and the cAMP waves which organise Dictyostelium development (Tyson & Murray, 1989; Siegert & Weijer, 1992).
In a relaying excitable medium there is a local rest state, which can be excited by pushing the state away from equilibrium, over a certain threshold. When this occurs, the state makes a wide detour through state space before returning to the rest state. Because of the influx of an activator into the nearby medium (usually by diffusion), a wave of excitation can form: when the medium is in the so-called excited state, it is able to push the nearby region over the threshold, which creates a propagating pattern of medium that is triggered into the excited state. Because the excited state is followed by a recovering state, during which the medium cannot be excited, such waves of excitation can only propagate forwards. In two-dimensional (2D) excitable media patterns are often organised in the form of spiral waves. Usually, spiral waves originate either because of inherent heterogeneity in the excitable tissue, or because of some special initial conditions. Sometimes, however, spiral waves spontaneously break up (and/or new ones appear) in a homogeneous medium, without any special initial conditions, a topic which has received a great deal of attention during the last few years.
The other class of excitable media consists of oscillatory excitable media. In these systems, there is no stable rest state; instead the local dynamics are in a limit cycle regime, called relaxation oscillation. Both classes, however, share many features, such as wave propagation, spiral waves, some types of breakup, and curvature-effects. Nevertheless, there are also differences. For example, in oscillatory systems target waves can form. These are waves that originate from a point source and spread out in an ever larger circle. Both classes of systems are closely related; in many models the behaviour can switch from one regime to the other as a result of only small parameter changes. In our module of Dictyostelium cAMP signalling we incorporate both types of systems in the one model, simply by making a small parameter change.
Many models converge to excitable media, and therefore one expects to find excitable media in many different biological settings. Note, however, that whereas many different models can behave as excitable media, the size of parameter regions, for which different types of behaviour can be found, varies between models. Hence, the behaviour that we observe in only a very small parameter region of one model can be the prime behaviour in another model. Hence, it can be a good heuristic method to zoom in on interesting parameter regions of models that are easier to study.
A well-known mathematical caricature for excitable media is the partial differential equation (PDE) model that is based on the FitzHugh-Nagumo (FHN) system (FitzHugh, 1961; Nagumo et al., 1962; FitzHugh, 1960). The FHN equations were originally developed as a simplification of the classical model for action potentials (Hodgkin & Huxley, 1952), but they have been widely used elsewhere. The model has two variables; one variable describes the excitation, and the other is the recovery variable. The equations exist in many variations; in this thesis we use the piecewise linear `Pushchino' version (Panfilov & Pertsov, 1984). Figure 3.1 shows the phase plane and dynamics of this model for both the excitable and the oscillatory regime. Because this model is a qualitative generalisation of excitable media, it can be used to describe waves in the BZ-reaction (see chapter 2), in the heart muscle, (Panfilov & Hogeweg, 1993), as well as in Dictyostelium (Savill & Hogeweg, 1997).
In this thesis we use excitable media in two different ways. In part I of this thesis we study a homogeneous excitable medium. We explore the parameter space of the above model in our search for spiral breakup and other interesting behaviour. We find several new types of patterns, hitherto unknown. The study illustrates that excitable media is a rich mechanism for pattern formation.
In part II of this thesis we apply the concept of excitable media to the process of Dictyostelium discoideum morphogenesis. Because we couple the excitable and motile properties of Dictyostelium cells, the pattern formation is now able to change both the shape and the excitability of the medium itself.
In order to describe the individual amoebae in our model we use the Glazier & Graner-model formalism, which is a two-scale cellular automata (CA) with differential adhesion. Steinberg (1963) first proposed the so-called differential adhesion hypothesis, asserting that differential intercellular adhesion is one of the most important factors in cell sorting. Differential adhesion, however, is not as clear a generic concept for pattern formation as is the concept of excitable media. This is mainly due to the fact that it is only recently that models have been developed that are really able to describe the basic elements of cell sorting arising from differential adhesion.
Many of the attempts to model cell sorting using standard CA models succeeded only when special tricks were used (for a thorough overview of such early model work on cell sorting, see Mostow, 1975). The main problem was that no ways could be found to overcome the local optima in the CA, which consist of many small clumps of cells, and arrive at global cell sorting.
The problem was finally solved by Graner & Glazier (1992); Glazier & Graner (1993). They presented a two-scale stochastic CA which is indeed able to generate complete cell sorting. Their model is called the extended large-Q Potts-model, or simply the Glazier & Graner-model. The precursor of the model dates from 1925, when Ising proposed a simple lattice model in which individual elements modify their behaviour so as to conform to the behaviour of other individuals in their vicinity. The Ising-model was proposed to explain certain empirically observed facts about ferromagnetic materials. In the Ising-model, each lattice site can be in one of two different states. Potts (1952) extended the Ising-model by assuming that each lattice site can be in one of q different states (where q > 2; for q = 2 both models are equivalent). The Potts-model was originally developed to simulate order-disorder transformations and surface-energy-driven diffusion in non-biological patterns. Initially, the focus was mainly on the statistical dynamics of the q = 3 and q = 4 Potts-model. It is only recently that researchers have begun to look at the spatial dynamics of the large-Q Potts-model, which has a much higher number of states, and in which a group of the same state refers to an individual soap bubble or `cell' (Glazier et al., 1990).
To be more concrete, the large-Q Potts-model is
embedded in a 2D lattice, but can easily be extended to three
dimensions (3D). It describes a collection of Q `cells' in that
each cell is given a unique identification number,
![]() = 1, 2,..., Q. A cell
= 1, 2,..., Q. A cell ![]() consists of all sites in the
lattice that have value
consists of all sites in the
lattice that have value ![]() . Bonds between different cells are
given an energy of 1 and bonds within one cell an energy of 0, and
then the total free energy is calculated. At each step a lattice site
is chosen at random and the chance that the state of one of its
neighbours will be copied into it is either 1 if
. Bonds between different cells are
given an energy of 1 and bonds within one cell an energy of 0, and
then the total free energy is calculated. At each step a lattice site
is chosen at random and the chance that the state of one of its
neighbours will be copied into it is either 1 if
![]() H
H ![]() 0 or
e
0 or
e![]() -
- ![]()
![]() if
if
![]() H > 0,
where
H > 0,
where ![]() H is the change in energy if the copying were to
occur. The pattern which evolves minimises surface area. Hence, large
compact cells are formed, while many others completely disappear. The
dynamics and statistics can, for example, describe accurately
surface-energy-driven grain growth, e.g. in soap froth
(from Graner & Glazier, 1992).
H is the change in energy if the copying were to
occur. The pattern which evolves minimises surface area. Hence, large
compact cells are formed, while many others completely disappear. The
dynamics and statistics can, for example, describe accurately
surface-energy-driven grain growth, e.g. in soap froth
(from Graner & Glazier, 1992).
Nevertheless, significant applications of this model-formalism in
biology only became possible after Glazier & Graner had made
two important extensions to the Potts-model. These
extensions were made to describe cell sorting resulting from
differential adhesion in biological systems. First,
Glazier & Graner introduced a second scale in the
model. They understood that biological cells have a more or less fixed
size, and they added to the free energy function an extra term, to
describe the area constraint for each individual cell (except for the
surrounding medium, of course). Secondly, they introduced different
contact energies between cells of different types by introducing a
second label, ![]() , which indicates to which cell type each cell
belongs, and by defining
J
, which indicates to which cell type each cell
belongs, and by defining
J![]() ,
,![]() , the surface energy
between two lattice sites occupied by cell types
, the surface energy
between two lattice sites occupied by cell types ![]() and
and ![]() .
.
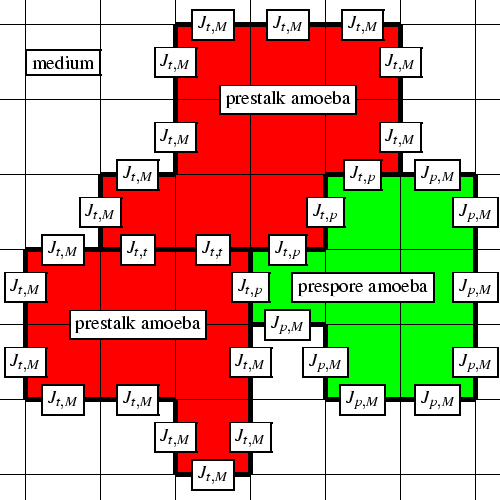
Figure 1.1 shows the model in a schematic way. Three different cells from two different cell types are shown, with their boundaries and contact energies. When the above extensions are taken into account, the Hamiltonian energy function becomes:
where v
 |
In part II of this thesis we use the Glazier & Graner-model to describe the individual cells of Dictyostelium. However, we again extend the model-formalism by adding dissipation costs to cell deformations and by including chemotaxis in the energy function. In last two chapters we make two more extensions, namely variable target volumes and cell-type transitions.
As stated earlier, we use the cellular slime mould Dictyostelium discoideum as a paradigm system for wide-ranging coordination during the multicellular stage of an organism. Much research has been done on Dictyostelium. In this section we describe both the experimental and theoretical background.
The slime moulds belong to the kingdom of Protista. Although the phylogenetic position of the slime moulds has been a source of confusion, several analyses suggest a divergence before the plant, animal and fungal clade, but late in the evolution of the Protista (Kessin, 1997).
The primary habitat of Dictyostelium is forest soil. Under normal conditions the cellular slime mould consists of unicellular amoeboid cells that move around and feed on bacteria. They themselves are predominantly preyed upon by nematodes (Kessin et al., 1996). When food becomes scarce, the amoebae enter a stage of aggregation. This leads to the formation of a multicellular organism that eventually forms spores, which are dispersed and able to survive during harsh conditions. During the multicellular development all amoebae are still self-sufficient. When completely disorganised and offered fresh bacteria, the amoebae promptly return to the vegetative stage and consume the available bacteria; when disorganised in the absence of bacteria, the same multicellular development promptly arises again (Raper, 1940).
Aggregation is the first stage of this fascinating process of morphogenesis. Initially the amoebae begin to communicate by emitting cyclic adenosine monophosphate (cAMP) signals, which are transmitted from amoeba to amoeba in the form of waves of excitation: Amoebae respond to stimulation by cAMP with an increase in cellular cAMP synthesis and subsequent secretion. The cAMP receptors, however, become desensitised, which terminates the cell response. Afterwards, the cAMP is degraded by phosphodiesterase, and returns to its resting level. The cAMP receptors become sensitive again, and the amoeba can again be stimulated. Diffusion of cAMP through the medium causes the signal to be relayed in space; the relayed signal takes the form of the observed macroscopic cAMP waves (Höfer et al., 1995b). Thus, the cAMP waves in Dictyostelium are a particular instance of wave patterns in excitable media (Tyson & Murray, 1989).
The spatial pattern of these waves is usually rather complicated. It consists of many rotating spiral waves, or of target waves that are generated by high-frequency pacemakers at their centre. The patterns already show a high amount of self-organisation, whereby more or less equal amounts of amoebae aggregate to one centre, a property which is only weakly dependent on cell density. Different strains show different `preferences' for either spirals or target waves during development, although strain behaviour is essentially interchangeable, for example, by adjustment of humidity (Kellerman & McNally, 1999).
The amoebae respond mechanically to the chemical signal via chemotaxis and start moving in the direction of the cAMP gradient. After propagation of 20-30 waves of cAMP, the amoebae collect in coalescing aggregation streams containing about 104 to 106 cells; then they collect in a compact aggregate, which is called the mound. Thereupon they begin to separate into two major cell types, prestalk and prespore cells. The prestalk cells sort out to the top of the mound and form a tip2. As soon as the tip is formed, the mound starts to elongate and forms a long thin structure, which is called the first finger. This structure usually tips over and forms a slug, which starts to migrate towards the soil surface. The prestalk cells occupy the anterior 30%, while the prespore cells occupy the posterior part; the whole slug is surrounded by a slime sheath. The slug senses the environment for cues such as light incidence, temperature and pH gradients and air streams, in order to orientate itself towards the surface. When the slug comes into a suitable environment for fruiting body formation, the tip reaches up, and the prespore cells crawl in underneath: the so-called culminant is formed, which initially is more or less hemispherical. Now the prestalk cells start to form the stalk, which elongates downwards through the cell mass to anchor itself to the base, while the prespore cells move upwards. These peculiar cell movements have been referred to as the ``reverse fountain''. In this way, the fruiting body is eventually formed, with a spore-filled globule at the top of a thin tapering stalk. At the base the so-called basal disc is formed, which gives the organism its scientific name; the spore head is surrounded by an upper and lower cup. Finally, to close the life cycle, the spores are dispersed and germinate again as unicellular amoebae.
There is yet another morphogenetic pathway, namely sexual development, which is accompanied by cannibalism. This occurs in the dark, usually under wet conditions. Mating begins with the appearance of tiny amoebae that move about much more rapidly than their larger vegetative counterparts. Shortly after their appearance, these gametes fuse in pairs to form binucleates, or sometimes even multinucleates. Within each binucleate the nuclei swell, migrate together and fuse. As this occurs, the cytoplasm increases in volume, so that once the nucleus forms, the cells are extremely large and are referred to as zygote giant cells. These giant cells secrete cAMP to attract the non-zygotic amoebae to their surfaces. Upon arrival these amoebae are ingested in a kind of cannibalistic phagocytosis. Chemotaxis occurs more rapidly than phagocytosis and therefore a zygote giant cell becomes surrounded by hundreds of cells. Once the zygote has ingested all of them it secretes a cyst wall before entering a period of dormancy. At germination meiosis occurs, and subsequent mitoses result in the production of many amoebae that emerge from the cyst (O'Day, 2000).
Some mechanisms, such as the cAMP signalling, are used in both pathways. It is often considered strange that the stalk cells give up their self-sufficiency during fruiting body formation. The mechanism of cannibalistic sexual development, however, puts this event in a different light. The sexual pathway is believed to have evolved before the fruiting body (Kessin, 1997), although this is still highly speculative. Although the sexual pathway has many interesting features, during the rest of this thesis it will nevertheless be completely ignored (as is normally the case when Dictyostelium is used as a model organism).
A number of key elements can be observed as the slime mould develops into a fruiting body. Here we describe the ones we think are most essential, and which we use in the full model. We discuss both the experimental observations and indicate how we have implemented them.
Motility is a complex process that depends on the coordination of many cellular functions, including the conversion of information from the environment into a series of coordinated responses that culminate in directed cell movement. Motility is one of the most important processes in Dictyostelium morphogenesis. Motion is essential during aggregation, the mound stage, slug motion and formation of the fruiting body, and is involved in all kinds of processes, such as cell sorting. But obviously it is also needed during the unicellular state, for grazing bacteria and evading nematodes.
Motility is implemented by using the Glazier & Graner-model, and stems from membrane deformations, which are governed by something akin to energy minimisations and which take cell adhesion, chemotaxis and volume conservation into account.
Adhesion is essential for normal development in many respects (Fontana, 1995). Three forms of cell adhesion determine the life cycle: adhesion of bacteria to the surface of the growing amoebae, as the prerequisite for phagocytosis; cell-substratum adhesion, necessary for both locomotion of the amoebae and migration of the slug; and cell-cell adhesion, essential for transition from the unicellular to the multicellular stage, since multicellular organisms cannot exist unless they are able to adhere to each other (Bozzaro & Ponte, 1995). Differential cell adhesion plays a role in many processes during all stages of the development, such as cohesion, cell sorting, and contact-mediated regulation of gene expression. Nowadays, many different cell adhesion molecules have been identified and purified.
The Glazier & Graner-model that we use has been developed to
describe differential cell adhesion. Differential adhesion is
implemented in the model by varying the surface contact energies
J![]() ,
,![]() .
.
The cAMP dynamics in Dictyostelium can be regarded as a very interesting realization of excitable media. During all stages, the motion of amoebae is orchestrated by waves of cAMP, which are formed by a combination of a pulsatile cAMP excretion and a cAMP-mediated cAMP response. This is accompanied by a chemotactic response towards cAMP. The field of aggregating amoebae is an unusual instance of pattern formation in excitable media, because cAMP is not only the substrate that forms the waves of excitation, generated by separate amoebae which receive and transmit the cAMP signal, but it is also an attractant for chemotactic motion of the amoebae. So, after excitation, the amoebae enter a phase of motion, during which the distance between the amoebae alters. Therefore, the next wave of cAMP will propagate through an excitable medium that differs slightly from the previous wave. Thus, in this system the wave of excitation changes the excitable medium itself. The velocity of motion of amoebae is low compared to the velocity of the wave of cAMP, and if we are interested in the short-time behaviour of this system, we can neglect the motion of the amoebae. However, if we want to model the process of aggregation itself, then the motion of the amoebae and its effect on the waves of excitation must be taken into account. The same holds for the later stages of the morphogenesis, since the slug also behaves like a moving 3D excitable system: slug motion has been shown to be organised by a scroll wave in the tip which breaks up into plane waves (Siegert & Weijer, 1992,1991). Although both spiral waves and target waves are observed during the aggregation, it is still not certain whether the slug motion may also be organised by target waves that move backwards from the tip. On the one hand, Kellerman & McNally (1999) observed that strains which show target patterns during aggregation often skip the whole slug stage and culminate instantaneously. When conditions are altered so that such a strain also shows spiral waves, the chances of slug migration also increase, which indicates that a scroll wave is important for the slug stage, at least for its initiation. On the other hand, Bonner (1998) managed to develop an experimental method for producing migrating 2D (one-cell-thick) slugs which share most basic properties with normal 3D slugs. Obviously, these slugs do not have a scroll wave. In chapters 3 and 4 we model migrating 2D slugs organised by target waves. The cAMP waves, combined with chemotaxis, are again essential during the culmination stage, where they organise the upward motion and, at the same time, force in a roundabout way the downward elongation of the stalk (see chapters 5 and 6).
In our model we use a mathematical caricature of the cAMP dynamics, namely the FHN system with piecewise linear `Pushchino' kinetics (Panfilov & Pertsov, 1984). The chemotaxis is implemented by taking the local cAMP gradient and by using this value in the energy function that drives the membrane-rearrangements in the Glazier & Graner-module.
A number of other signalling molecules besides cAMP also play a role during development. One of these key molecules is ammonia (NH3). The developmental roles of NH3 have been frequently underestimated: NH3 regulates the onset of aggregation, controls the timing of differentiation, functions as a morphogen for cell proportioning, determines whether or not the slug stage will be skipped completely, is connected to thermotaxis, phototaxis and acidotaxis during slug migration, regulates the timing of switching from migration to culmination, determines fruiting body orientation, and is linked with the final sporulation. For an overview, see Cotter et al. (1992). Dictyostelium is certainly not the only organism which uses NH3 as a long-distance communication signal. For example, NH3 also mediates communication between yeast colonies (Palková et al., 1997).
In our model we use a PDE to describe the production and turnover of NH3, which depend on factors like the local light intensity3 and the presence of charcoal in the medium. NH3 alters the excitability of the cells. This is implemented by modifying the parameter value that defines the excitability in the excitable medium module.
The amoebae differentiate into two major cell types, as well as many other (sub) cell types. The cell cycle phase at the onset of starvation imposes a relative preference for a specific cell fate. However, when prestalk cells or prespore cells are separated, they take on almost normal cell proportions within a few hours. The most important morphogens that control cell differentiation and transitions between cell types are: cAMP, required for both prestalk and prespore differentiation; differentiation-inducing factor (DIF), which induces prestalk and inhibits prespore differentiation; and NH3, which antagonises DIF (Schaap et al., 1996; Williams, 1988; Kay, 1997).
We do not implement cell differentiation in a detailed way in our model: it is assumed that cells never change their major cell type. The only cell-type transitions we use are to describe the maturation of prestalk cells, which changes cell adhesion, as well as properties connected with excitability. For this process we simply assume local cell induction by a non-specified process.
From late aggregation onwards the multicellular mass is covered in a thin extracellular matrix, which contains mainly protein and cellulose (Smith & Williams, 1979). This is called the slime sheath. During slug migration, slime is continuously synthesised, and a trail of slime is left behind. It is unclear whether all cells in the slug contribute to the production; most of the slime may in fact be secreted by the outer layer of cells (Wilkins & Williams, 1995). Different cell types may also produce different amounts and elements of the slime sheath. The slime sheath plays an important role in slug formation, maintenance of the morphology and migration.
The extracellular matrix which surrounds the stalk, the so-called stalk tube, is quite different. This matrix is produced by the stalk cells, and is of a different composition, which makes it much stiffer. Its prime role is to strengthen the stalk that supports the spore head. In chapter 6 we show that it may also play a role in the downward elongation of the stalk.
The extracellular matrices are implemented as very large cells in the Glazier & Graner-module. The differences in stiffness are described by differences in the dissipation costs involved in changing their shape.
In the early seventies, Dictyostelium discoideum attracted the attention of theoreticians, and since then the organism has become a paradigm for theories of pattern formation during development. Three aspects make Dictyostelium very suitable for this task: the relative simplicity of the patterns; an almost invariant development over a huge range of total cell numbers; and a remarkable capacity to restore proportions, patterns and morphogenesis when disturbed and fragmented (Nanjundiah, 1997).
Various aspects of the development have been studied mathematically, in roughly the same order as the morphogenesis itself. Already in the forties, Bonner (1949,1947) showed that both the aggregation and the later stages are organised by a signalling molecule which functions as a chemoattractant; he called the molecule acrasin, but it later turned out to be cAMP (Barkley, 1969). Very early in the seventies authors of several theoretical studies tried to explain the initiation of the aggregation in terms of breakdown of stability (Nanjundiah, 1973; Keller & Segel, 1970; Cohen & Robertson, 1971b,a). These studies showed that production and decay of a chemoattractant can lead to unstable modes, which could possibly be interpreted as aggregation and streaming. In 1973, however, Durston was the first to recognise that Dictyostelium aggregation fields behave as an excitable medium. He focused mainly on how the macro-scale phenomena could be understood in terms of an excitable medium. Alcantara & Monk (1974) were the first to elucidate the possible biological mechanisms by which the system behaves as an excitable medium.
The mechanism of cAMP signalling has been modelled in detail by Martiel & Goldbeter (1987). This model is based on experimental studies and describes the interaction between cAMP and its membrane receptor (this mechanism was first introduced by Segel et al., 1986). The model takes into account both the desensitisation of the cAMP receptor by reversible phosporylation and the activation of adenylate cyclase that follows binding of extracellular cAMP to the unmodified receptors. By assuming that all the reversible binding steps in the kinetic model are rapid, these authors have shown that the model reduces to three coupled nonlinear ordinary differential equations: the fraction of a receptor in the active state, the intracellular cAMP concentration and the extracellular cAMP concentration. All the parameters in the reduced model are composites of many processes. The numerical solution of the model agrees in quantitative detail with experimental observations of the period, amplitude and form of the cAMP oscillations, as well as with the experimental observations of the adaptation to constant cAMP stimuli and the absolute and relative refractory period.
Their study, however, did not take the spatial geometry into account. To establish the effective diffusion coefficient ( Deff) of cAMP during aggregation, which was ignored in the above-mentioned study, Foerster et al. (1990) measured the velocity-curvature relation which they used to calculate Deff. To obtain both the detailed time- and space-characteristics of the cAMP signal, Dallon & Othmer (1998) modelled the precise cAMP dynamics of two nearby cells. See also the work by Othmer & Schaap (1998), who, in an attempt to elucidate the detailed dynamics, carry the modelling to an extreme.
The first attempts to model the spatial patterns of the aggregation stage were made by Parnas & Segel (1977,1978). They modelled aggregation using a one-dimensional (1D) grid, and showed that periodic cAMP signalling combined with chemotaxis leads to very efficient aggregation. MacKay (1978) used a 2D grid. His model used discrete cells that could be in different states and a number of transition rules that describe the cell-to-cell signalling. The model showed propagating waves of cell movement and aggregation in coalescing streams.
The model developed by Martiel & Goldbeter (1987), reduced to two variables, was implemented for the first time in a spatial context by Tyson & Murray (1989) and by Tyson et al. (1989), in order to study waves of excitation in a uniform 2D field of signal-relaying but immobile cells. To obtain propagation these authors added a diffusion operator to the equation that describes the extracellular cAMP. They showed that this model describes in quantitative detail experimental observations of rotating spiral waves of cAMP in fields of amoebae distributed over an agar surface. More recently, different mechanisms have been proposed to explain the formation of spiral waves during early aggregation (Lauzeral et al., 1997; Levine et al., 1996; Pálsson & Cox, 1996).
Using the Martiel & Goldbeter-model, Levine & Reynolds (1991) calculated that the stream formation could be due to an instability of the combined signalling-chemotaxis system. At the end of 1994 and the beginning of 1995, two studies appeared which, for the first time, really considered the interplay between the actual cell aggregation process and the cAMP dynamics. Vasiev et al. (1994) used an FHN-type model for the cAMP dynamics and a continuity equation for the amoebae motion, whereas Höfer et al. (1995b); Höfer et al. (1995a) derived a PDE model with intracellular and extracellular cAMP, as well as cell density. Both models were able to reproduce the cellular pattern formation during aggregation. These two studies, however, offered different explanations for the streaming instability. According to Höfer et al. (1995b); Höfer et al. (1995a) stream formation is due to a chemotaxis-driven instability4. Vasiev et al. (1994) on the other hand showed that stream formation can be due to a density-dependent wave speed, i.e. cAMP waves travel faster in regions with higher cell density. van Oss et al. (1996) elaborated this idea by combining the Martiel & Goldbeter-model for cAMP signalling with a discrete cell description. They showed that in the Martiel & Goldbeter-model, stream formation due to the dependence of wave speed on cell density occurs only when the turnover rate of intracellular cAMP is high.
The regulation of cell type proportions was modelled by Schaap et al. (1996). They showed that differential production and turnover of cAMP, DIF and NH3, and their effects on cell differentiation, produce more or less correct proportions of the different cell types. The subsequent cell sorting was modelled by Sekimura & Kobuchi (1986) in a CA which incorporated both differential chemotaxis and differential cell adhesion. They found, in contrast to the differential adhesion hypothesis (Steinberg, 1963), that differential adhesion should operate in such a way that prestalk and prespore cells intermingle: in their model, small clumps of cells only slow down the sorting, which is driven completely by the chemotaxis. However, this can be regarded as a model artefact, since, as was indicated in section 1.3, CA models are not able to give a good description of cell sorting caused by differential adhesion. Umeda (1989) constructed a mixed-fluid model, and showed that cell sorting could be due to differential chemotaxis towards cAMP only. Glazier & Graner (1993) developed the two-scale CA model described in section 1.3, and showed that cell sorting patterns, as observed in submerged agglomerates of Dictyostelium (Takeuchi et al., 1988), could be explained adequately by differential intercellular adhesion only (see also Graner, 1993; Graner & Sawada, 1993; Graner & Glazier, 1992). Cell-adhesion-driven cell sorting versus chemotaxis-driven cell sorting is still a source of debate (see Clow et al., 2000, and the discussion in section 7.3.2). The Glazier & Graner-model formalism was used by Savill & Hogeweg (1997) to describe aggregation and mound formation (as well as the crawling slug). They made two important extensions to the model: (i) they added chemotaxis to the model by adding another layer with cAMP dynamics and using the local, subcellular cAMP gradient in the energy function; and (ii) they extended the model to 3D, so that it became possible for the first time to follow directly the transition from the aggregation stage to the mound stage. They showed that not only chemotaxis and wave propagation, but also cell adhesion play an important role in stream formation. They also showed that streams can be functional, because streams move faster than individual cells.
Further 3D work on the mound stage was done by Bretschneider et al. (1997). They combined discrete cells with the Martiel & Goldbeter-model and showed formation and elongation of the mound, as well as cell sorting by differential chemotaxis. Levine et al. (1997) and Jiang et al. (1998) have also studied the mound stage, albeit in less sophisticated models. Finally, Dormann et al. (1998) combined an FHN-model for the cAMP dynamics with a Navier-Strokes equation for the cell motion. They showed that cell sorting provides feedback to the wave geometry. This eventually leads to the formation of a twisted scroll wave, which later dominates the dynamics during the slug stage.
The first model for the mechanism underlying slug motion was constructed by Odell & Bonner (1986). They proposed that the forward motion may be achieved by a fountain-like circulation of cells. In the mixed fluid model of Umeda (1989), a continuous chemotactic motion forwards was assumed, leading to a number of very peculiar slug shapes. Both models, however, contradict experimental observations (Siegert & Weijer, 1992,1991). On the basis of these experiments, Steinbock et al. (1993) showed that in a tube-shaped excitable medium with decreasing excitability (which is a caricature for a slug), a twisted scroll wave in the highly excitable prestalk zone breaks up into plane waves in the less-excitable prespore zone. Bretschneider et al. (1995) showed the same conversion of scroll waves into planar wavefronts in the more realistic Martiel & Goldbeter-model. Bretschneider et al. (1999) have added to this model the explicit motion of the cells, represented as spheres, while taking both cell-type specific chemotaxis and adhesion into account. The model created stable scroll waves that were able to generate coordinated slug migration. On the other hand, the above mentioned model developed by Savill & Hogeweg (1997) showed that target waves, which originate in the tip and move through the slug, can also drive a multicellular slug, when these are combined with chemotaxis and adhesion.
In contrast to the other stages, the culmination stage has not yet been modelled in terms of biological processes, although the shape changes of the culminant during the culmination have been modelled (Zeeman, 1977; Rubinow et al., 1981).
In part II of this thesis we study the interactions of the slug with the environment and we describe how the cellular slime mould morphogenesis culminates in the formation of a fruiting body.
In part I of this thesis we study the general problem of complex pattern formation in a homogeneous excitable medium. In part II we couple the pattern formation mechanism to a CA module which describes the cell dynamics, in order to develop a comprehensive model of Dictyostelium discoideum morphogenesis. We use this as a paradigm to study multicellular coordination and development. The particular chapters are organised as follows:
In chapter 2 we study the phenomenon of spiral breakup. Nowadays it is generally accepted that heart-fibrillations and sudden cardiac death are caused by the occurrence and breakup of spiral waves. This has triggered a whole range of research on the origin of spiral waves and the mechanisms behind spiral breakup (Panfilov & Holden, 1997). The formation of spirals and their breakup not only play a role in heart diseases, but they are also important in Dictyostelium development, and in chemical systems, such as the BZ-reaction.
In earlier models, the mechanism which breaks up spirals was connected to the meandering of the spiral tip (Bär & Eiswirth, 1993) or related to spatiotemporal instabilities of the wave train, which even occur in a 1D case (Karma, 1994,1993; Panfilov & Hogeweg, 1993). It turned out that there is a simple criterion for this instability, which can be formulated in terms of the restitution curve. Both mechanisms lead to breakup close to the core. The new mechanism for breakup elucidated here specifically explains patterns observed in the BZ-reaction (Markus et al., 1994; Markus & Stavridis, 1994a,b), but is expected to reflect a much more general principle. We show that due to a high diffusion rate of the inhibitory variable the wavefront can become laterally unstable. Due to this high diffusion rate, the wave velocity is restrained by the high level of inhibitor ahead of the wavefront. However, the influx of the inhibitor into the region ahead of the wavefront is smaller in the case of a convex wave than in the case of a straight wave. As a result, the convex wave can propagate faster. Therefore, microscopic variations in the wavefront will be enlarged, which can potentially lead to spiral breakup.
We show that we can zoom in on an interesting parameter region by using a genetic algorithm. The parameter region in which we find spiral breakup is at the edge between wave-like phenomena and (quasi) stable patterns. This is because high enough diffusion rates of an inhibitor generally lead to Turing-like patterns. Along this edge a number of other interesting patterns can be found, such as, for example, oscillatory behaviour that is driven solely by diffusion.
In part II we present a model for Dictyostelium development, and show that the morphogenesis can unfold as a result of the interplay between excitable media and differential adhesion, and the feedback between levels of organisation.
In chapters 3 and 4 we look at the slug stage. The model for excitable media from the previous chapter is combined with a stochastic two-scale CA which describes cell rearrangements caused by differential adhesion. We link these modules to each other by adding chemotaxis to the CA, using the local cAMP gradient (see also Savill & Hogeweg, 1997).
Chapter 3 falls into two sections. The first part describes the slug motion. The model slugs maintain their shape and crawl, with a velocity depending on slug size, as is found in experiments. We also show that the interaction between differential adhesion and chemotaxis, which is the same for all cells (in contrast to the claims made in earlier work), can explain fast and complete cell sorting.
In the second part we look at the thermotactic behaviour of the slug. We show that thermotaxis can be generated in our model by means of the collective behaviour of the amoebae, whereas individual amoebae can neither sense a temperature gradient, nor show temperature-dependent differentiation in motion velocity. Instead, differences in temperature alter the excitability of the cells, and thereby the shape of the cAMP wave. Chemotaxis towards cAMP then causes the slug to turn towards the temperature gradient. It is very striking that the mechanism still functions at extremely low signal-to-noise ratios. This is possible because the cAMP waves behave like spatiotemporal signal integrators.
In chapter 4 we focus on another property of the slug that directs it to the soil surface, namely phototaxis. We add an extra CA module, in which we use a ray-tracing model to describe light refraction, and a PDE which describes NH3 dynamics. We also include the effect that NH3 has on the cAMP dynamics. Light rays are refracted and focused on the side of the slug that is opposite to the light source. Light increases NH3 production, which decreases excitability. As reported in the previous chapter, this changes the shape of the cAMP waves, and the slug turns towards the light source. However, when the sensitivity to NH3 is too high, the final direction of motion is at a certain angle with respect to the light source. This can explain the behaviour of mutants that also show so-called bi-directional phototaxis. When the light intensity is lower, or when NH3 is absorbed by `charcoal', the bi-directionality decreases or disappears, as is found in experiments. Our mechanism can explain not only phototaxis, but also orientation in a pH gradient (acidotaxis) and towards an air current (rheotaxis). These two types of orientation are due to an interference with the NH3 balance: along a pH gradient the NH3/NH4+ ratio changes, and an air current carries NH3 away.
In the final two chapters we study the final stage of Dictyostelium, namely the culmination that leads to the formation of the fruiting body. The movement during the culmination has been likened to a ``reverse fountain'', because prestalk cells in the upper part form a stalk which moves downwards and anchors itself to the substratum, while prespore cells in the lower part move upwards to form the spore head.
In chapter 5 we extend the model further with extracellular matrices, i.e. the slime sheath and stalk tube, and we introduce differences in stiffness. We also include cell differentiation generated by some kind of induction process. We show that the whole process of culmination can unfold by the interaction of cAMP signalling, differential adhesion, cell differentiation and production of extracellular matrices. Periodic upward movements towards the cAMP waves induce pressure waves, which squeeze the stalk downwards through the cell mass. The mechanism creates an elongated stalk; downward motion stops automatically when the base is reached and upward motion stops once the stalk formation has finished; and finally the spore head becomes rounded. Moreover, the mechanism is also self-correcting; for example, any deviations in the downward elongation of the stalk are quickly restored. A high number of experimental observations are in agreement with the mechanism we propose. The mechanism can, for example, explain the well-known phenomenon of two culminants orienting away from each other.
In chapter 6 we look in greater detail at the mechanisms behind the culmination. More specifically, we look at the detailed requirements for the different subprocesses and study how they interact with each other. We show that it is not sufficient for stalk elongation to have only a (stiff) tube or only pathfinder cells; both are needed, since it is only in interaction that an elongated stalk can be created and that the resistance caused by the upward moving cells can be efficiently reduced, which makes the fast downward elongation possible.
We also look at the essential requirements for the large number of surface tensions between all the cell types. This gives us an indication of the ranking one expects to find in experiments on binding strengths. We end this chapter by connecting side-effects observed in the model with experimental observations. It is possible that our model may be able to provide an explanation for the array of aberrant phenotypes that can be created nowadays by mutation generating experiments.
Thus, the chapters of part II together demonstrate that it is feasible to model morphogenesis in a sense that goes well beyond pattern formation. Such an approach can be used to study how a facultative multicellular organism can sense the environment, on many different scales, and exhibit a coordinated response.
We have identified a whole set of mechanisms by means of which Dictyostelium is able to coordinate morphogenesis. These mechanisms are reviewed in chapter 7. In the last chapter we also look in greater detail at the modelling approach we have used. And finally we will point to the gaps in our knowledge and give suggestions for further research.